Ten Years of Dedication
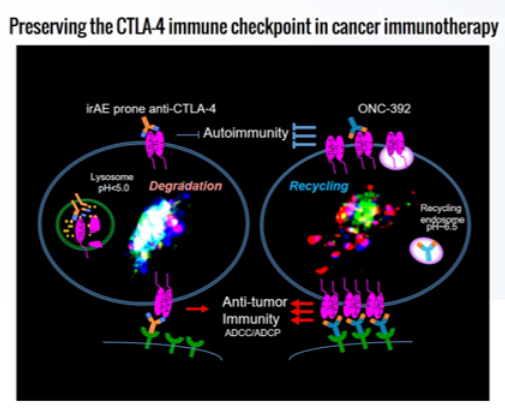
· Characteristics:A new generation of anti-CTLA-4 monoclonal antibodies, capable of most effectively and selectively eliminating regulatory T cells in the tumor microenvironment, which is a major culprit in tumor immune evasion;
· Differentiation:Unlike traditional anti-CTLA-4 antibodies, ONC-392 does not induce lysosomal degradation of CTLA-4, thereby preserving the immune tolerance checkpoint function of CTLA-4 in other parts of the body.
· Therapeutic Advantage:Compared to other CTLA-4-targeting drugs, ONC-392 significantly improves the therapeutic index.
2022.04 Monotherapy for PD(L) 1-resistant non-small cell Lung Cancer (NSCLC)
received Fast Track designation from the U.S. FDA
2023.02 Phase 1b clinical trial for monotherapy in the treatment of gastrointestinal solid tumors officially launched at the Sun Yat-sen University Cancer Center
2023.03 Germany BioNTech collaborates with OncoC4 (AcroImmune)
$200 million upfront payment + milestone payments + double-digit percentage of sales royalties
2023.06 Overseas: International multicenter Phase III clinical trial initiated
2025 Overseas: BLA in 2025
2025 China: BLA; preparation for industrialization

